The process of electrolysis uses electricity to separate elements like the ones in water – a molecule made up of two hydrogen atoms and one oxygen atom. Sometimes referred to as an alternative fuel, today, a handful of car designs use the process of electrolysis as fuel instead of traditional gasoline options.
Follow the instructions below, and see if you can perform electrolysis and separate your water’s molecules into hydrogen and oxygen. Let’s do science!
What you need:
• Glass jar filled with water
• Disposable cup or paper disk
• Two pencils, sharpened at both ends
• Two alligator clips
• Nine-volt battery
• Table salt
 What you do:
What you do:
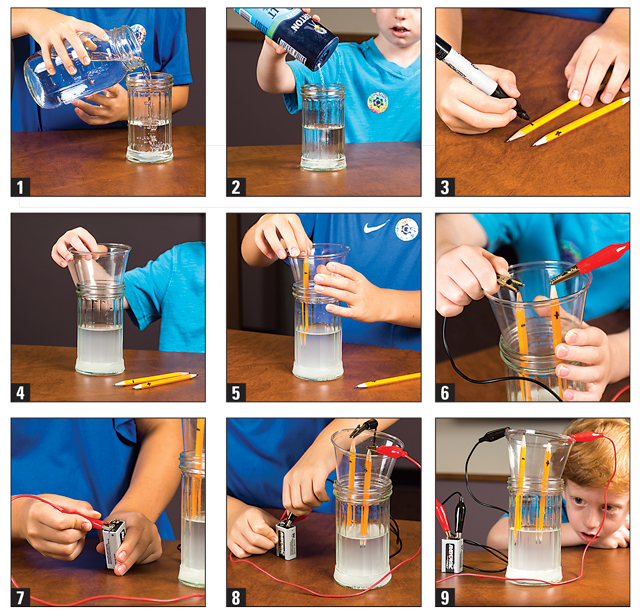
1. Fill a jar about three-fourths full with water.
2. Add about three tablespoons of salt.
3. Label your pencils: one with a negative sign at the top, and one with a positive sign at the top.
4. Place the disposable cup on the top of the jar.
5. Poke your two pencils through your cup so that the tips of the pencils are under water
on one side.
6. Attach one alligator clip to the top of one pencil, and the other alligator clip to the top
of the other pencil.
7. Attach the pencil with the negative symbol at the top to the negative side of the nine-volt battery.
8. Attach the pencil with the positive symbol at the top to the positive side of the nine-volt battery.
9. Observe what’s happening. What are the bubbles doing? Record your findings.
 Science challenge:
Science challenge:
See what happens when you switch out the pencils and replace them with nails. Try the experiment without salt. What’s the difference?